Biliary Atresia Treatment Market Set for Robust Growth, Projected to Reach USD 1.64 Billion by 2032
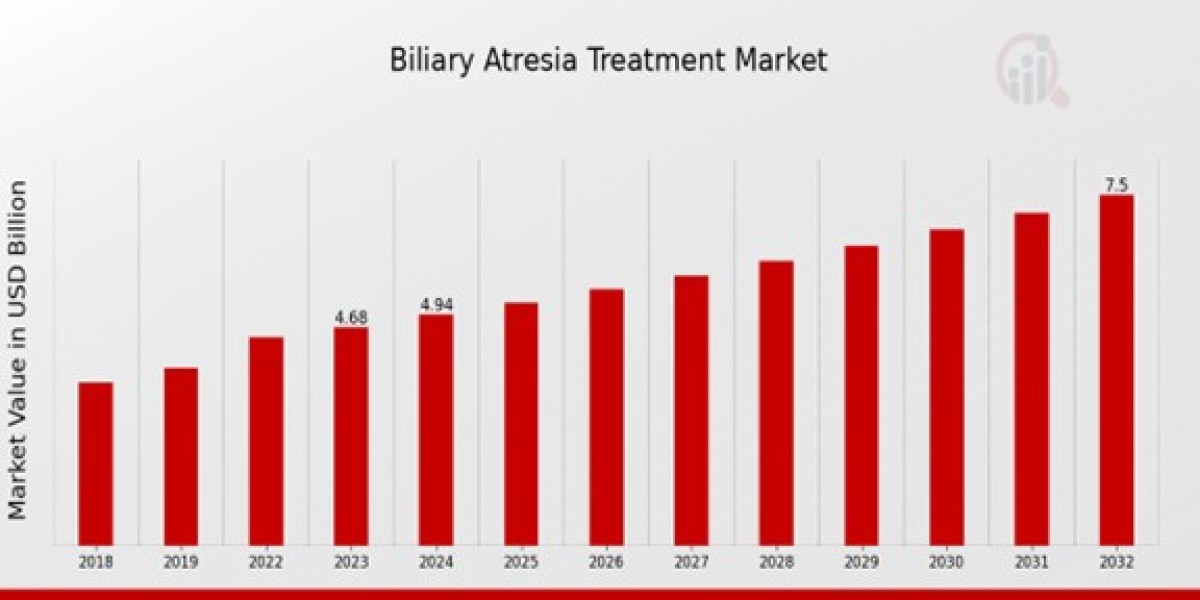
The global Biliary Atresia Treatment Market is experiencing significant growth, driven by increasing awareness, advancements in treatment options, and a rising number of diagnosed cases. According to a new report from Market Research Future (MRFR), the market, valued at USD 0.97 billion in 2023, is forecast to expand at a compound annual growth rate (CAGR) of 5.18% to reach USD 1.64 billion by 2032.
Market Overview
Biliary atresia is a rare but severe liver condition affecting infants, characterized by the obstruction or absence of bile ducts, leading to liver damage. The disease's growing incidence is the primary driver behind the demand for advanced treatment options. With significant advancements in surgical techniques, pharmacological therapies, and the emergence of stem cell treatments, the biliary atresia treatment landscape is evolving rapidly.
Key Trends:
- Rising adoption of minimally invasive surgical techniques, such as laparoscopic and robotic surgery
- Increased awareness about the disease through media, patient advocacy, and social media channels
- Development of personalized treatment methods and telemedicine integration
- Advancements in stem cell-based treatments
Market Scope and Segmentation
The Biliary Atresia Treatment Market is segmented based on treatment type, age group, severity, pathophysiology, and treatment setting.
Treatment Type: The market is divided into Kasai procedure, liver transplant, and medical management. The Kasai procedure, which serves as the first line of treatment, is expected to hold the largest market share, followed by liver transplants for end-stage liver failure.
Age Group: Neonates are expected to dominate the market share due to the higher incidence of the disease in this group. Infants and pediatric patients are also significant segments, with a strong growth trajectory driven by early diagnoses.
Severity: Severe cases of biliary atresia require complex surgical interventions, positioning the severe segment as the largest contributor to market growth. Moderate cases are also witnessing growth due to increased early diagnoses.
Pathophysiology: The market is predominantly driven by idiopathic biliary atresia, followed by cases linked with other liver diseases and genetic syndromes.
Treatment Setting: Hospital-based treatments are expected to dominate in 2023 due to the specialized care required. However, outpatient-based settings are gaining traction with the rise of minimally invasive techniques.
Regional Insights
- North America is leading the market with over 40% of the global revenue share, backed by high healthcare standards and prevalence rates.
- Europe follows as the second-largest market, while Asia-Pacific (APAC) is poised for rapid growth due to rising healthcare spending and increasing awareness.
- South America and the Middle East & Africa are emerging markets with substantial growth potential.
Key Market Drivers and Developments
The key drivers fueling growth in the biliary atresia treatment market include the increasing prevalence of the condition, technological advancements in treatment options, and the growing focus on patient awareness. Minimally invasive surgical methods and innovative therapeutic approaches are expected to propel market expansion.
Recent Developments:
FDA Approval of MINI-ACCESS™ Biliary Stent System by Boston Scientific for use in pediatric patients with biliary atresia marks a significant advancement. The system offers a less invasive, effective treatment option, enhancing patient outcomes and reducing complications.
Companies like Medtronic and Stryker continue to invest in research and development, contributing to advancements in surgical products, including stents and catheters, which aim to improve the lives of biliary atresia patients.
Key Market Players
The competitive landscape of the Biliary Atresia Treatment Market includes several prominent companies such as:
- Boston Scientific Corporation
- Medtronic
- Stryker Corporation
- Edwards Lifesciences
- Abbott Laboratories
- Olympus Corporation
- Johnson & Johnson
- Zimmer Biomet
- Braun Melsungen AG
These companies are focusing on expanding their product portfolios, forming strategic alliances, and conducting research to develop advanced treatment solutions for biliary atresia. Mergers and acquisitions are common in this dynamic market, as companies aim to strengthen their market positions and expand their geographic presence.
Conclusion
The global Biliary Atresia Treatment Market is on an upward trajectory, propelled by technological advancements, increased awareness, and an evolving treatment landscape. As the prevalence of biliary atresia continues to rise, stakeholders in the healthcare and pharmaceutical industries are expected to benefit from the increasing demand for effective and minimally invasive treatment options.
For more insights on the Biliary Atresia Treatment Market, request a free sample or access the full report.
About Market Research Future (MRFR)
Market Research Future (MRFR) is a global market research company that provides comprehensive, actionable insights into various markets, including healthcare, technology, and chemicals. Our reports help clients make informed decisions about their business strategies and investments.
Contact Information: Market Research Future (MRFR)
Phone: +1 646 845 9312
Email: sales@marketresearchfuture.com
Website: www.marketresearchfuture.com