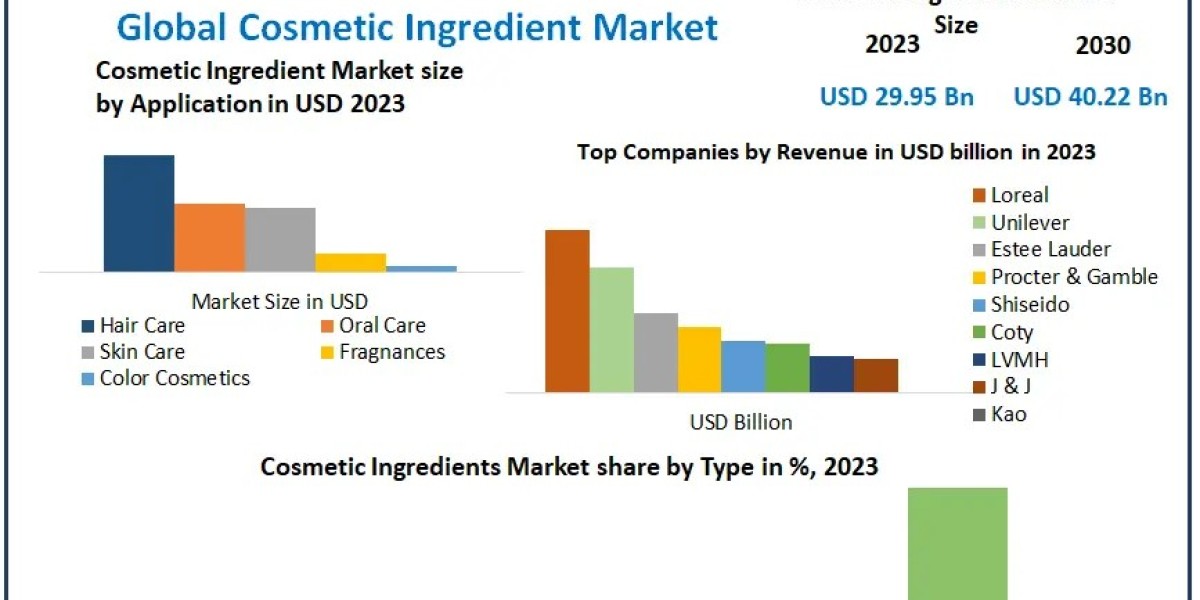
In the realm of healthcare, pharmaceutical companies stand at the forefront of innovation, striving to develop groundbreaking treatments and medications to improve patient outcomes worldwide. Behind every successful pharmaceutical endeavor lies a carefully crafted organizational structure designed to optimize research, development, manufacturing, regulatory compliance, and commercialization efforts. In this blog, we delve into the intricate structures of pharmaceutical companies, exploring the key components that contribute to their success.
The Foundation: Research and Development (R&D)
At the heart of every pharmaceutical company lies its Research and Development (R&D) division. This is where groundbreaking discoveries are made, and potential drug candidates are identified and nurtured. R&D departments typically consist of multidisciplinary teams of scientists, researchers, and clinicians who collaborate to explore new therapeutic targets, conduct preclinical studies, and advance promising compounds through clinical trials.
Clinical Operations
Once a potential drug candidate shows promise in preclinical studies, it progresses to the clinical stage, where its safety and efficacy are evaluated in human subjects. Clinical operations departments oversee the planning, execution, and monitoring of clinical trials, ensuring compliance with regulatory requirements and ethical standards. This involves coordinating with clinical research organizations (CROs), investigational sites, and regulatory authorities to conduct studies efficiently and ethically.
Regulatory Affairs and Compliance
Navigating the complex landscape of regulatory requirements is paramount for pharmaceutical companies seeking to bring their products to market. Regulatory affairs departments play a crucial role in ensuring compliance with local and international regulations governing drug development, manufacturing, and marketing. These teams work closely with regulatory agencies such as the FDA (Food and Drug Administration) in the United States or the EMA (European Medicines Agency) in Europe to obtain approvals and licenses for new drugs.
Manufacturing and Supply Chain
The manufacturing and supply chain functions are integral components of pharmaceutical operations, responsible for producing, packaging, and distributing medications to patients worldwide. Manufacturing facilities must adhere to stringent quality standards and Good Manufacturing Practices (GMP) to ensure the safety, efficacy, and consistency of pharmaceutical products. Supply chain management involves coordinating raw materials procurement, production scheduling, and distribution logistics to meet demand and minimize supply chain disruptions.
Commercialization and Marketing
Once a drug successfully navigates the regulatory process and receives approval, it enters the commercialization phase, where marketing and sales efforts come into play. Commercialization teams develop strategic marketing plans, conduct market research, and engage with healthcare professionals, payers, and patients to promote awareness and adoption of the new medication. Sales teams work to secure formulary placement, negotiate pricing, and drive prescription volume to achieve revenue targets.
Corporate Functions
Supporting the core operations of pharmaceutical companies are various corporate functions, including finance, human resources, legal, and information technology. These departments provide essential services such as financial management, talent acquisition, legal compliance, and IT infrastructure to ensure the smooth functioning of the organization.
Collaboration and Partnerships
In an increasingly interconnected world, pharmaceutical companies often collaborate with academic institutions, research organizations, biotechnology firms, and other industry stakeholders to advance scientific knowledge and accelerate drug discovery and development. Collaborative partnerships enable companies to leverage complementary expertise, share resources, and mitigate risks associated with drug development.
Conclusion
The organizational structure of pharmaceutical companies is a complex tapestry of interconnected functions and departments, each playing a vital role in bringing life-saving medications to patients around the globe. From research and development to manufacturing, regulatory affairs, commercialization, and beyond, every aspect of the pharmaceutical enterprise is carefully orchestrated to drive innovation, ensure compliance, and improve patient outcomes. As the healthcare landscape continues to evolve, pharmaceutical companies will remain at the forefront of innovation, pushing the boundaries of science and medicine to address unmet medical needs and improve the quality of life for millions of people worldwide.